Clinical Studies
M6-C™ Artificial Cervical Disc Two-Level IDE Clinical Study
This study is currently enrolling patients with degenerative cervical radiculopathy requiring surgical intervention, confirmed clinically and radiographically, at 2 adjacent vertebral levels between C3 and C7.
Clinical Studies
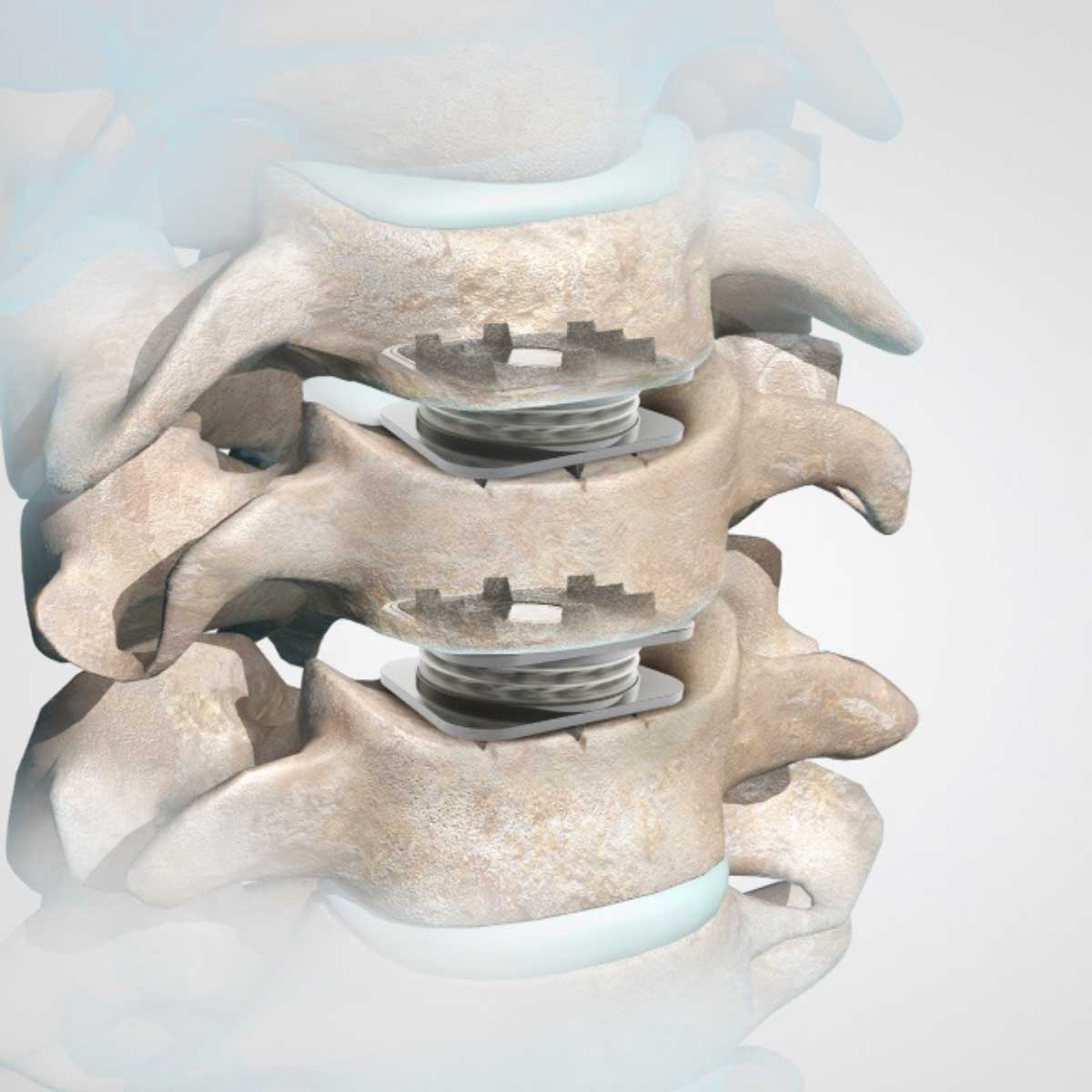
M6-C™ Artificial Cervical Disc Two-Level IDE Clinical Study
This study is currently enrolling patients with degenerative cervical radiculopathy requiring surgical intervention, confirmed clinically and radiographically, at 2 adjacent vertebral levels between C3 and C7.
The study’s primary objective is to evaluate the safety and effectiveness of the M6-C™ artificial cervical disc compared to anterior cervical discectomy and fusion (ACDF) for treating two-level symptomatic cervical radiculopathy at vertebral levels from C3 to C7 with or without spinal cord compression.
You may be eligible for the study if:
- Have been told you need neck surgery at two adjacent levels between C3 to C7
- Are experiencing neck and/or arm pain after at least 6 weeks of conservative, non-surgical treatment
- Are willing and able to attend follow-up progress visits with your doctor over 24 months and possibly 5 years
- Do not have any autoimmune disorders, cancer, and not insulin-dependent diabetic
- BMI < 45
- Are between 18 years and 75 years of age
For more information and resources, please visit:
ClincialTrials.gov (study Identifier NCT104982835).
